Medical school professor Cho Mi-ra identifies protein with potential to treat refractory systemic...
- Writer :External Relations Team
- Date :2025.02.10
- Views :63
CUK’s medical school professor Cho Mi-ra identifies protein with potential to treat refractory systemic sclerosis
CUK’s medical school professor Cho Mi-ra identifies protein with potential to treat refractory systemic sclerosis
- Discovered the fibrosis-inhibiting effect of the mitochondrial transport protein "GRIM-19"
- Opens up new possibilities for treatment of systemic sclerosis

[Image: (From left) Professor Cho Mi-ra, Research Professor Park Jin-sil, Professor Park Seong-hwan]
Professor Cho Mi-ra from the Department of Pathology at Catholic University of Korea Medical School (co-corresponding author), Research Professor Park Jin-sil from the Catholic Rheumatism Research Center (co-first author), Researcher Jeong Ha-yeon from the Department of Biomedical Health Sciences (co-first author), and Professor Park Seong-hwan from the Department of Rheumatology at Catholic University of Korea Seoul St. Mary's Hospital (co-corresponding author) announced that the mitochondrial transport protein “GRIM-19” (GRIM-19; the retinoid-IFN-induced mortality-19) is a new mechanism that inhibits disease progression in systemic sclerosis.
This research has proved the therapeutic effect of GRIM-19 in systemic sclerosis by explaining the mechanism of protein regulation for pathogenic immune cell suppression and mitophagy regulation in fibroblasts. It provides crucial scientific grounds for understanding the pathological mechanism and establishing effective treatment strategies.
Systemic sclerosis is a refractory autoimmune disease where fibrosis develops not only in the skin but also in major organs such as the lungs and heart. This disease currently has no fundamental cure, causing great hardship for patients. This disease can also affect capillaries, leading to skin thickening and hindering blood circulation. Moreover, the development of pulmonary fibrosis can cause breathing problems, which can even be fatal.
The research team examined the correlation between STAT3, a transcription factor that increases during progression of systemic sclerosis, and GRIM-19 protein, which inhibits the activity of STAT3 and is involved in the mitochondrial function. In a mouse model where systemic sclerosis was induced, the research team saw an increase in the expression of fibrosis markers alpha-SMA, collagen type 1 (Col I), and STAT3, and a decrease in the level of GRIM-19 protein. This result suggests that there is a link between the fibrosis process and the GRIM-19 protein.
The research team performed gene therapy to increase expression of the GRIM-19 protein, observing that the thickness of the dermal layer of the skin decreased and the expression of inflammatory cytokines (TGF-beta, IL-6, IL-17, IL-1beta) and fibrotic proteins (a-SMA, Col1) also decreased in mice with induced systemic sclerosis. In addition, reductions were also observed in the pathogenic autoimmune cells Th2 and Th17.
The GRIM-19 protein was shown not only to inhibit the activity of STAT3 but also to play a role in facilitating “mitophagy,” a process in which damaged mitochondria are removed. Through cell experiments, the research team confirmed existence of the mechanism by which the GRIM-19 protein increases transport of STAT3 into mitochondria, suppressing fibrosis
In a disease model of systemic sclerosis, it was observed that overexpression of GRIM-19 resulted in smoother removal of damaged mitochondria and a reduction in the expression of fibrosis-related proteins. This suggests that GRIM-19, by regulating mitochondrial function, could be utilized as a potential therapeutic strategy for systemic sclerosis.
Professor Park Seong-hwan stressed that while systemic sclerosis is a typical refractory disease for which drug development is difficult, his research team has established a patient avatar model that enables development of more accurate targeted therapies. He also added that if the GRIM-19 gene therapy, which induces mitochondrial transport, can be applied to patients after preclinical evaluation, it will become a highly promising therapeutic candidate.
Professor Cho Mi-ra said, “In refractory immune diseases, activation of the STAT3 transcription factor and mitochondrial dysfunction have been observed in immune cells and lesion sites. The GRIM-19 protein, identified in this study, can simultaneously regulate these problems, making it a very important treatment target for preventing or overcoming refractory fibrotic diseases.”
This study has shown the crucial role that the mitochondrial transport protein GRIM-19 plays in systemic sclerosis. It not only inhibits the transcription factor STAT3, reducing immunopathogenesis and fibrosis, but also improves mitochondrial function and activates mitophagy by transporting STAT3 into the mitochondria, thereby leading to the treatment of systemic sclerosis.
Therefore, GRIM-19 was identified as a protein that can play an important role in treatment of systemic sclerosis through inhibiting and regulating the JAK-STAT pathway, which is one of the important mechanisms in systemic sclerosis, and at the same time, through increasing STAT3 in mitochondria to restore mitophagy, removing damaged mitochondria.
This research was published in the internationally renowned journal in the field of immunology, Experimental & Molecular Medicine (IF: 9.5), under the title "GRIM-19-mediated induction of mitochondrial STAT3 alleviates systemic sclerosis by inhibiting fibrosis and Th2/Th17 cells."

Figure 1. Correlation between fibrosis markers
and expression of GRIM-19 protein in the skin of systemic sclerosis patient
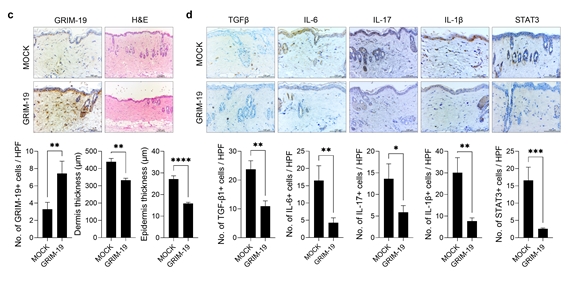
Figure 2. Inhibition of fibrosis through overexpressing the mitochondrial transport protein GRIM-19 in systemic sclerosis
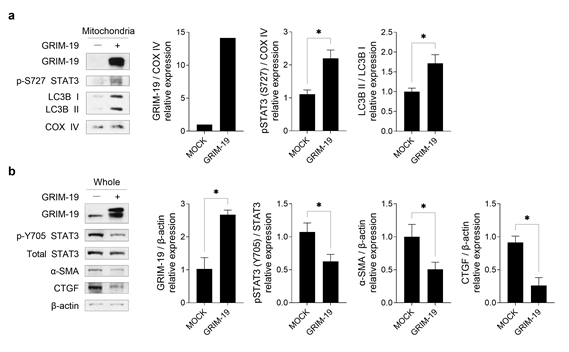
Figure 3. Progression of mitophagy and suppression of fibrosis after overexpression of the GRIM-19 protein in fibroblasts

